Just Enough Genetics
Just Enough Genetics to Understand Your Pathogenic Gene Variant
What is a gene?
A gene is a tool in a system called DNA, which produces proteins. The system generates over 100,000 proteins that work together to make your body function, doing jobs from carrying oxygen to moving muscles. When you eat protein, your body breaks it into pieces, and DNA helps reassemble those pieces into your own proteins.
Each protein has a particular job and is made with a specific gene. For example, a gene called LDLR makes a protein called an LDL receptor that transports cholesterol in the blood. A gene called BRCA1 makes a protein that helps repair damaged DNA and prevent tumors, among other tasks. Your DNA contains all the genes to make the complete set of proteins your body uses. In fact, it has two copies of most genes. You inherit one copy from each of your biological parents.
Your mother has two of each gene, too—one from each of her parents. She passed on only one to you. So did you get your LDLR gene from your grandfather or your grandmother? It could be either. You have a 50% chance of inheriting each copy. You have the same random chance when you inherit genes from your father, so your DNA contains a mix of genes from all four grandparents.
If both copies of a gene make the same protein, does it matter which grandparents you got them from?
Even though your copies of each gene produce the same protein, the versions of the gene are different.
The gene a child gets isn’t always an exact duplicate of their parent’s. The inheritance process can go wrong. The gene may be missing sections or have extra parts. These changes are called mutations. Once a person inherits a changed version of a gene, their children have a 50% chance of inheriting it, like any other gene. The versions, or variants, can be passed down for generations.
All your genes are likely to have changed at some point. The BRCA1 variant you inherit from your mother may have mutated 10 generations ago, while the BRCA1 variant from your father may have a separate mutation from 15 generations ago. Because the genes are not the same, the proteins they make may not be the same.
Most changes are not a big deal. Both proteins do their job just fine. Even if one version of a protein doesn’t work perfectly, you have backup—the other version picks up its slack. Mutations can even be good because they make people individuals. Every person's DNA has a unique legacy of changes. The proteins in one person don’t do their jobs exactly the same as the proteins in another person, so you don’t look or act like anyone else.
However, every once in a while, a variant is so mutated that its protein is terrible at its job. The protein does its best, but it’s likely to miss something important. A variant could also make proteins that don’t function at all. A variant that makes these kinds of proteins is a pathogenic variant.
When someone says their cancer risk is high because they have “the BRCA1 gene,” they mean that one of their copies of the BRCA1 gene is a pathogenic variant.
If you have a pathogenic variant, will you get cancer or other disease?
Having a pathogenic variant makes you more likely to develop disease than the general population, but it doesn’t guarantee you’ll get sick.
Sometimes, a pathogenic variant builds proteins that make small errors. Each mistake doesn’t matter much, but the consequences accumulate. If proteins that deal with cholesterol don’t work well, excess cholesterol can collect in the arteries and eventually lead to heart disease.
Proteins may also make a few big errors that are so bad they cause a disastrous chain of events. Or they may not be able to do their job at all. When a protein’s job is to fix problems that lead to tumors, like the protein made by BRCA1, key mistakes in the wrong places can let cancer take root.
Occasionally, the non-pathogenic variant gets damaged in one part of the body and starts producing defective proteins. The backup fails. If neither copy of a BRCA1 gene makes proteins that effectively do their job to prevent tumors, cancer can develop in that body part.
In many cases, though, the proteins made by the non-pathogenic copy of the gene can handle all the work on their own. In other cases, the proteins made by a pathogenic variant are good enough. They aren’t the best at their job, but they don’t make enough errors to cause disease.
About 50–80% of women who inherit one BRCA1 pathogenic variant develop breast cancer. In these women, proteins make enough mistakes to start a disastrous chain reaction that leads to disease. In the other 20–50% of women, the problems don’t get that far. Not everyone with a pathogenic variant develops its associated disease, a concept called incomplete penetrance.
Some people who don’t inherit a pathogenic variant will still get cancer. Proteins made by non-pathogenic variants, no matter how good they are at their jobs, can make mistakes or get damaged, too. Whether you have a pathogenic variant or not, your risk of disease increases with age because mistakes accumulate over time.
If you have a pathogenic variant, do you have control over whether you’ll eventually get sick?
It depends on the disease. Fortunately, you can lower your chances of developing some diseases through preventative actions.
Most people don’t find out they have a pathogenic variant until they already have the disease, when it’s too late for prevention. Learning you have a pathogenic variant before you develop a disease can be a shock, but you can use that knowledge to improve your odds of a healthy life.
With certain variants that carry a higher risk of heart disease, you can take statins to clean up some of the mistakes your proteins make.
With certain variants that carry a higher risk of cancer, you can get frequent screenings to catch a tumor early. You can remove organs to prevent the cancer starting.
Your doctor and genetic counselor can tell you the recommended prevention steps for your variant.
If a pathogenic variant runs in your family, wouldn’t you know about it?
Because not everyone with a pathogenic variant gets sick, you may have disease running in your family and not know about it. If your parent and grandparent carried a pathogenic variant of LDLR but never developed heart disease, you would have little reason to suspect you have a genetic propensity for cholesterol issues.
How can you find out if you have a pathogenic variant before you get a disease?
Doctors and genetic counselors can order tests to find variants in your DNA that are known to be pathogenic.
Usually a person gets a test because they’ve been diagnosed with a disease. If they are found to have a pathogenic variant, other relatives probably share the variant, too. A family health history can help identify which relatives are most likely to have the variant without knowing it.
A variant often appears on a family tree in a recognizable pattern that you can trace. Many, but not all, disease-associated genes are inherited in an autosomal dominant pattern, which looks like a branching line. For genes with incomplete penetrance, that line doesn’t trace who actually gets the disease. It traces who has a higher risk of the disease because they have a variant that can’t make working proteins.
Autosomal refers to the location of a gene in the DNA. Human DNA is packaged in 46 groups called chromosomes. Sex information is carried on two of those chromosomes, which are passed on with distinct patterns of inheritance. The other 44 chromosomes are autosomal and have their own pattern.
Dominant refers to the effect of an individual variant in a pair. Even if a person inherits only one dominant variant, it will impact their body. You don’t need both of your BRCA1 variants to be pathogenic for your cancer risk to rise—one is enough. Everyone who inherits a dominant pathogenic variant also inherits higher risk, and the variant from the other parent can’t do anything about it. This means that the line on a family tree has no gaps. Visible disease may skip a generation, but risk of disease does not.
Begin looking for an autosomal dominant pattern on a family tree with the first person to be diagnosed, called a proband.
The proband likely inherited the variant from a parent. If one parent has the disease, the variant probably comes from that side of the family. The parent may be able to get genetic testing to confirm this. If not, you could start with that assumption. Just be aware that the assumption may not turn out to be true. Draw the variant’s line of inheritance from the proband to the parent.
If one of the proband’s grandparents has the disease, they could have the variant, too. You may assume this or confirm it with tests. Continue the line through a parent to that grandparent. You can do this even if the proband’s parent doesn’t have the disease. The variant and its associated risk do not skip generations. Go ahead and fill in the line—it’s logical to conclude that a parent must have the variant to pass it to their child.
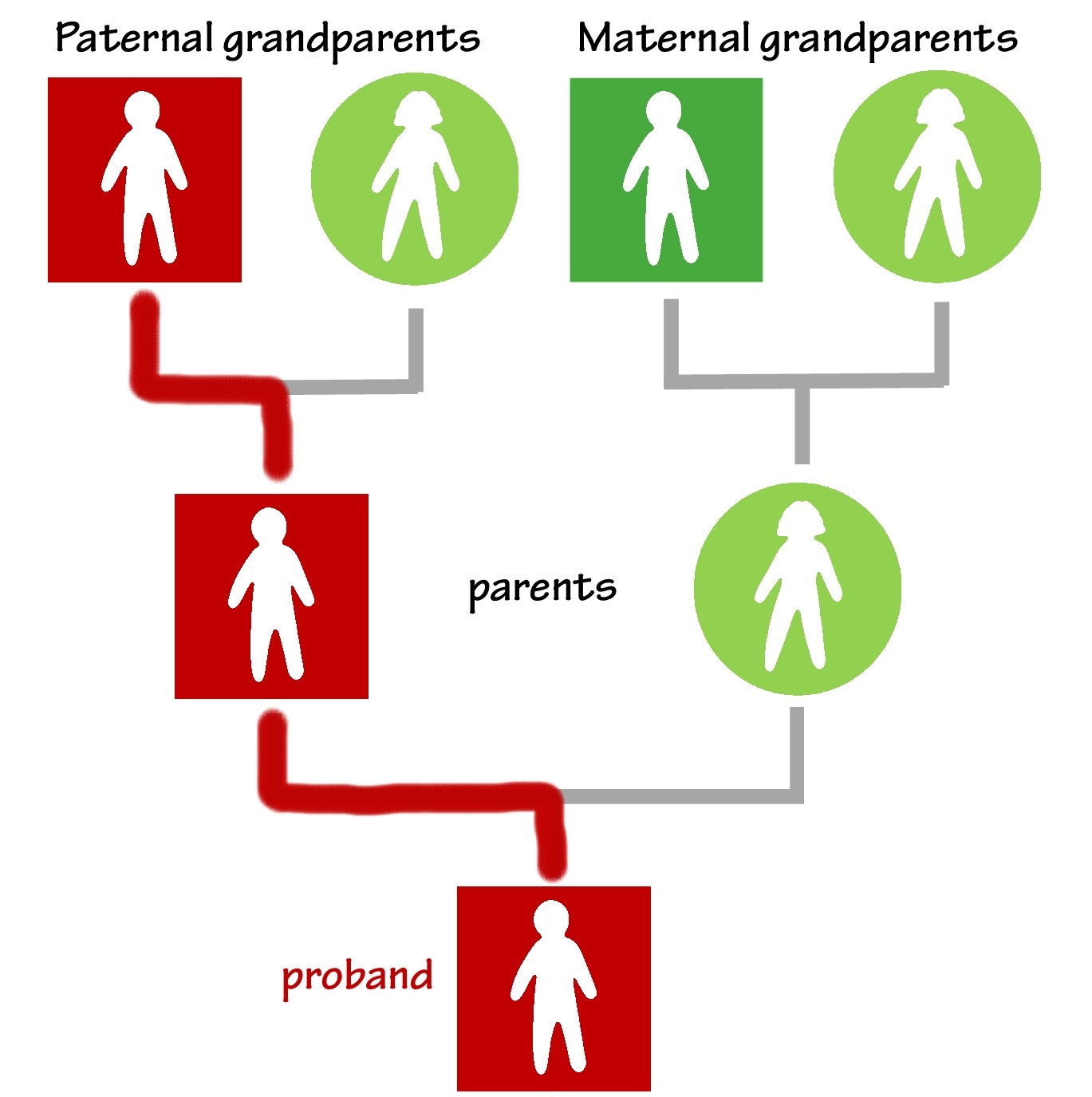
Every person along the line of inheritance—whether or not they have the disease—has a 50% chance of passing the variant to each of their children. Those children have the opportunity to ask their doctors about genetic testing while they still have options to prevent disease.
Draw branches of the line from the proband to any of their children who test positive for the variant.
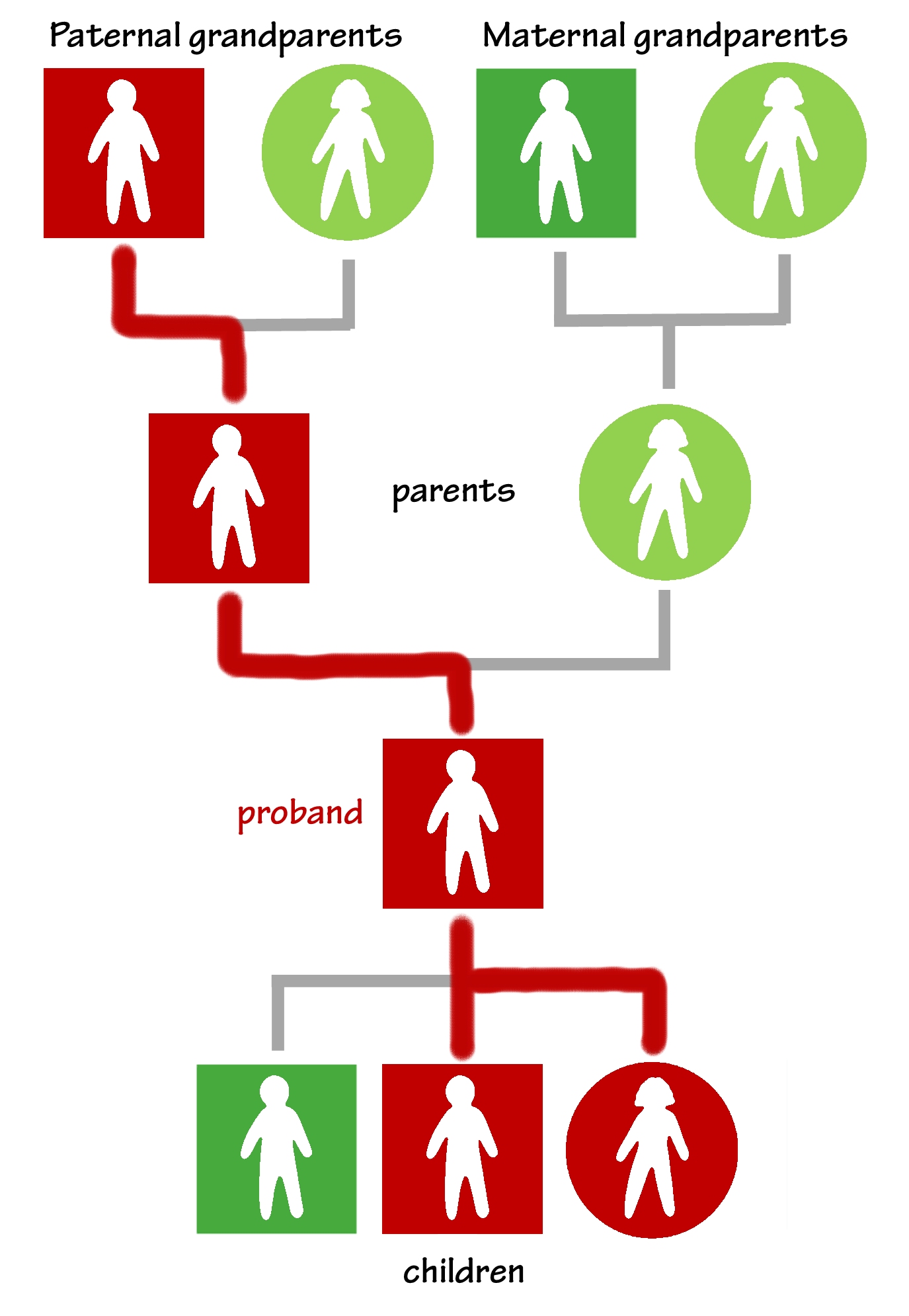
The parent’s children (the proband’s siblings) could have inherited it, too. If any learn they have the variant, draw branches of the line to connect them to the parent who passed it to them.
The grandparent’s children (the proband’s aunts and uncles) potentially carry it. If any test positive for the variant, draw more branches to show their inheritance.
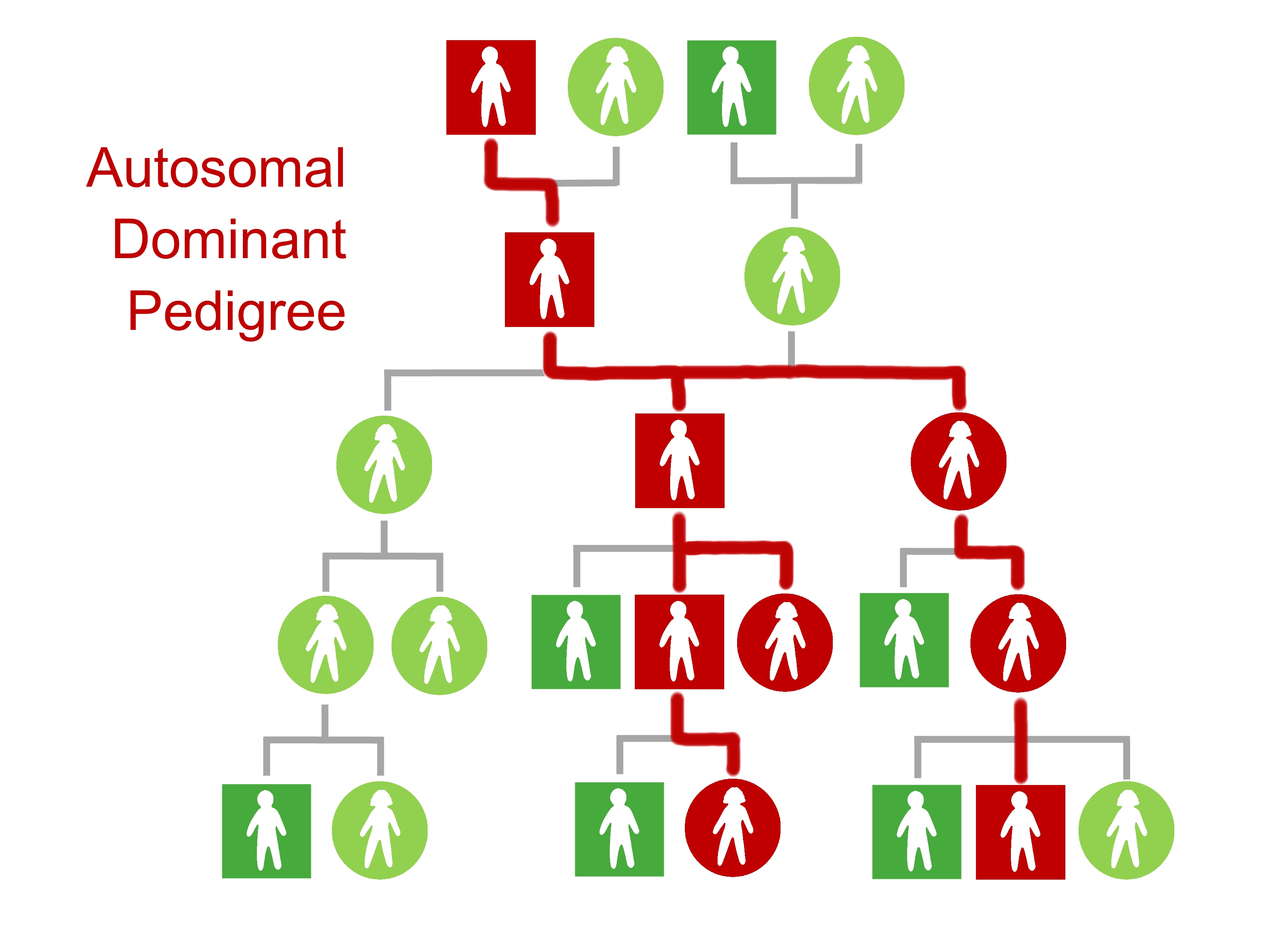
When each new person finds out they have the variant, they also find out that their children may have it, too. As those children choose to be tested, the branches spread. The line extends from the proband’s children to their grandchildren, from their siblings to their nieces and nephews, and from their aunts and uncles to their cousins.
More and more relatives learn that a pathogenic variant runs in the family, as well as where it runs. Those on the line can benefit from genetic testing to learn about their risk of preventable disease.
All test results, whether negative or positive, are empowering. Those who test negative know their branch stops with them. They won’t pass the variant to their children. Those who test positive can take steps to prevent disease. They can also encourage their children and grandchildren to get tested.
If not many of your relatives develop disease, can you still trace the line?
Tracing a pathogenic variant is most powerful in families where the variant has passed invisibly through a few generations. Members of these families are unlikely to know they carry the variant. But in these cases, tracing is more difficult. As disease appears to skip generations, the direction of the line of inheritance becomes unclear.
Clues in family health histories hint at where the branches of the line actually spread. When distant relatives in a family have the same variant, you may be able to figure out their connection. For example, if two second cousins are diagnosed with the same variant, you can assume that a great-grandparent they share has it as well. When you draw the line from each second cousin to their great-grandparent, you pass through a parent and a grandparent who must have the variant, too. With just two positive tests, the variant’s path of inheritance could become visible across three generations. The multiple cousins, siblings, aunts and uncles who connect to that path have a 50% chance of sharing the variant, and they can talk to their doctors about their disease risk.
The line gets muddied when relatives who don’t have the pathogenic variant develop disease. Maybe a parent with a pathogenic BRCA1 variant doesn’t have cancer, but the other parent, without the variant, does. Your first assumption of which of the proband’s parents and grandparents carry the variant could be wrong. The discovery of a great-uncle on the other side who has cancer may help you realize where you should redraw the line.
You don’t have to figure out the puzzle on your own. Family outreach navigators at ConnectMyVariant can help you trace the line of inheritance on your family tree and identify who is most likely to benefit from genetic testing.
What if you can’t trace an unbroken line of inheritance for the variant?
You don’t need to pin down every person on the variant’s line of inheritance. Even when the line has missing pieces, your relatives can still find out about their risk.
If you know that your grandparent has the variant, you may not know which of their children inherited it. Your first cousins could worry because they’re unsure whether their parent could have passed the variant on to them.
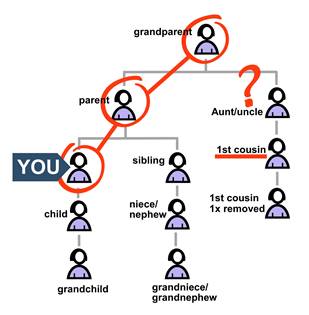
In this situation:
• Your first cousin knows that there is a 50% (one-half) chance that their parent inherited the variant.
But another chance is involved—this one with an “if” attached.
• If that parent inherited the variant, there is a 50% (one-half) chance your cousin inherited it, too.
Multiply the chance that you know by the chance with an “if.”
chance you know x chance with an “if” = actual chance
½ x ½ = ¼
Your first cousin has a one-fourth, or 25%, chance of carrying the variant. Medical guidelines usually suggest that people with a 25% chance of having a pathogenic variant should get genetic testing.
The further back you trace the variant in your family tree, the more relatives you can identify who could be at risk.
You may be able to identify which of your great-grandparents has a pathogenic variant. Your second cousin may wonder if disease risk runs on their side of the family. But, as far as they know, that great-grandparent is their only ancestor who definitely carries the variant.
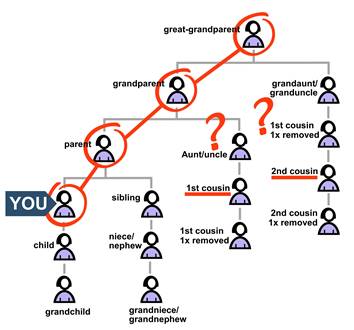
In this case:
• Your second cousin knows their grandparent had a 50% chance of inheriting the variant.
• If that grandparent inherited it, their parent had a 50% chance.
• If that parent also inherited it, your second cousin had a 50% chance.
Multiply the known chance by all the chances with “ifs.”
chance you know x chance with an “if” x chance with an “if” = actual chance
½ x ½ x ½ = 12.5%
Your second cousin has a 12.5% chance of carrying the variant. Medical guidelines usually suggest that people with a 12.5% chance of having a pathogenic variant should get genetic testing.
You may be able to trace the variant to a great-great-grandparent. You can then use the same formula to find your third cousin’s risk.
½ x ½ x ½ x ½ = 6.2%
Your third cousin has a 6.2% chance of carrying the variant. At this level, medical guidelines start to get less clear about genetic testing. Insurance may or may not pay for the tests. However, if your third cousins have a family history of disease in their parents, grandparents or other relatives and they find out about a known risk of inheriting a pathogenic variant, insurance will often cover genetic testing.
What if you can’t trace your line back very far?
Stay connected to your support systems. The places you already seek support are the same places you can find people who might expand your family tree. You may meet a person who has a missing piece of the puzzle.
You could find someone with your variant through ConnectMyVariant or in a social media support group. A person who shares your variant is likely to be your distant cousin. You probably inherited the variant from an ancestor you have in common. Family outreach navigators at ConnectMyVariant can help you try to identify your shared ancestor. If you can find this common ancestor, you don’t have to start with yourself and work backward in time through your family tree. Instead, you can start with the ancestor and work forward.
Keep talking with your family. You may run into a cousin who is in contact with another cousin you’ve never met. Your network may be larger than you think.
If you want to actively seek out relatives outside your support network, your family outreach navigator can help you use genealogy to discover the names of distant relatives. Or you might find relatives through DNA technology companies like Ancestry. When you approach a newfound relative, talk about your family stories and connection first, then share your knowledge about hereditary disease.
